Magnesium and graphite: an electricity experiment #science #chemistry #cathode #anode #physics

Informations de téléchargement et détails de la vidéo Magnesium and graphite: an electricity experiment #science #chemistry #cathode #anode #physics
Auteur :
Science CauldronPublié le :
17/12/2025Vues :
2.1KDescription :
Vidéos similaires : Magnesium and graphite

Урок №37. Необратимый гидролиз бинарных соединений и солей и некоторые ОВР

Reactivity of Alkali Metals with Water
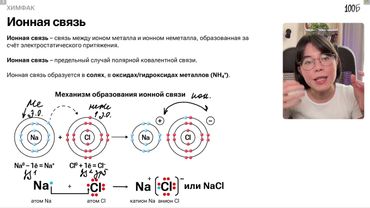
Урок №07 Ионная связь Металлическая связь Водородная связь

Урок №88. Водород. Кислород. Пероксид водорода

Урок №76. Алюминий, бериллий, цинк и их соединения

